ФІЗИЧНІ ПАРАМЕТРИ СИНТЕЗОВАНОЇ ГЕТЕРОМЕТАЛІЧНОЇ КОМПЛЕКСНОЇ СПОЛУКИ
Анотація
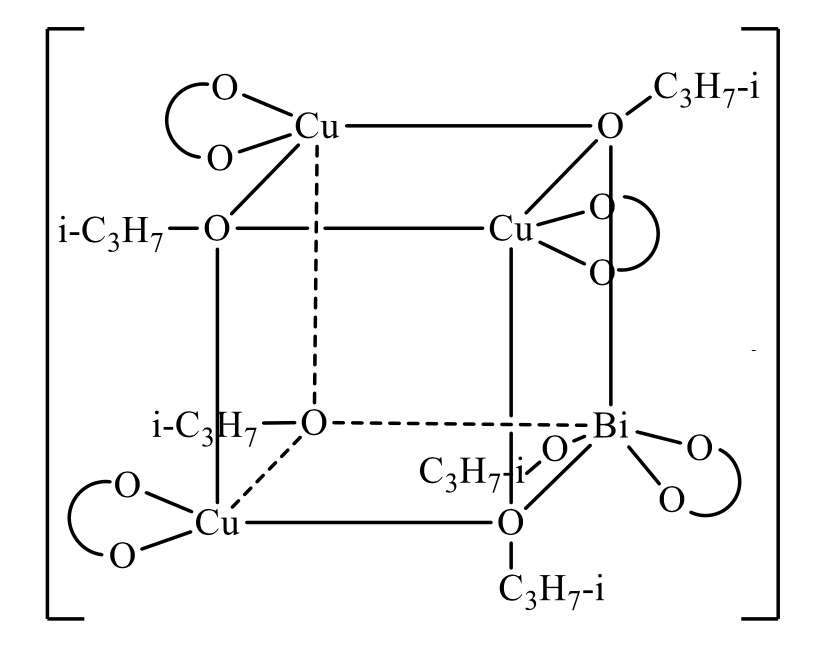
Розглянуто можливість використання нового синтезованого напівпровідникового матеріалу, як матеріалу чутливого до впливу температури або магнітного поля. Показано методику синтезу гетерометалічного µ-ізопропоксо (купрум(ІІ), бісмут(ІІІ)) ацетилацетонату (І). На основі даних елементного аналізу, ІЧ-спектроскопічних, магнетохімічних та термогравіметричних досліджень встановлено склад синтезованого гетерометалічного µ-ізопропоксо(купрум(ІІ), бісмут(ІІІ)) ацетилацетонату, який відповідає такій хімічній формулі:[Cu3Bi(С5Н7О2)4(OC2H5)5], де С5Н7О2 = H3C–C(O)–CH–C(O-)–CH3. Практичний вихід складає 82% від теоретично розрахованого. Виділена комплексна сполука (I), являє собою дрібнокристалічний порошок, який розчинний в суміші диметилформаміду з хлороформом (1:1), важко розчинний в спиртах, етері, краще розчиняється в диметилсульфоксиді, диметилформаміді, у воді руйнується. Аналіз отриманих експериментальних даних фізико-хімічних досліджень для сполуки (I) дозволив запропонувати схему розміщення хімічних зв’язків. Молекула гетерометалічного µ-ізопропоксо(купрум(ІІ), бісмут(ІІІ)) ацетилацетонату, вірогідно, являє собою куб, у вершинах якого розташовані три атоми купруму(ІІ) та один атом бісмуту(ІІІ), що з’єднані атомами оксигену ізопропоксо-груп, кожна з яких виконує роль тридентатного ліганда. Для виділеної комплексної сполуки [Cu3Bi(С5Н7О2)4(OC3H7-і)5], розраховано молярну масу, яка дорівнює 1090,5 г/моль та кількість валентних електронів в одній молекулі – 289. Для проведення експериментальних досліджень використовували циліндричний зразок масою 0,14 г та об’ємом 17,67∙10-9 м3, який виготовляли з комплексної сполуки (І) методом пресування. Експериментальні дослідження електропровідних властивостей µ-ізопропоксо (купрум (ІІ), бісмут (ІІІ)) ацетилацетонату в інтервалі температур 313 К – 413 К, в спресованому вигляді, показало, що при збільшені температури його питомий опір різко зменшується від 7·1010 до 4·102Ом·см. Інтервал робочих температур складає від +273 до +493 К, причому розкладання хімічної сполуки відбувається з 523 К, концентрація носіїв заряду зростає від 1,3·1019 м-3 при 273 К до 3,395·1036 м-3 при 493 К., при цьому квантова константа Холла при збільшенні температури від 273 К до 493 К зменшується від 0,566 м3·Кл-1до 2,167·10-18 м3·Кл-1, напруга Холла в діапазоні магнітного поля від 0 до 1000 мТ змінюється від 8,32·10-14 до 8,32·10-12 В.